Monday Feb 16, 2026
Monday Feb 16, 2026
Friday, 25 June 2021 01:30 - - {{hitsCtrl.values.hits}}
False negatives, with PCR and RAT, can also result from incorrect sampling, if the swabs are not inserted properly and swished around in the nose and throat so that enough viral proteins or virus particles stick onto the swab. This can give a false sense of security or assurance to the person who may go around spreading the virus – Pic by Shehan Gunasekara
 Some basic facts on the two testing methods available to identify the COVID-19 virus, would help us understand the measures taken by the health authorities to control the spread of the virus.
Some basic facts on the two testing methods available to identify the COVID-19 virus, would help us understand the measures taken by the health authorities to control the spread of the virus.
The virus causing the COVID-19 disease (called SARS CoV-2) should be unmistakably identified to not only manage patients but to also control the spread of the virus. As soon as the genome of the virus was made known by Chinese scientists in January 2020, tests were quickly developed to identify the virus. This test, popularly known as the PCR test, is a chemical reaction performed in a PCR machine under very strict laboratory conditions to avoid contaminations. It is more accurately called rT-PCR, which stands for reverse Transcriptase Polymerase Chain Reaction. The discovery of this reaction earned Kary Mullis, a maverick US scientist, the Nobel Prize for chemistry in 1993.
The genome of the virus is a long chain of four ‘letters’ of the genetic alphabet called RNA (most genomes, such as ours, are DNA). Using a combination of these letters, a complete set of instructions are available for the virus to gain entry into cells in our body, take over the machinery of the cell to make multiple copies of itself, which burst out of the cells to infect new cells. The genome consists of a very specific sequence of ‘letters’, which is peculiar to the COVID-19 virus.
These ‘letters’ are chemicals called bases. The bases are codes for amino acids which are assembled into proteins. Scientists have isolated two fragments from the genome of the COVID-19 virus (the genome is nearly 30,000 bases long), which are unique to only the COVID-19 virus and not shared with RNA from any other organism. These unique fragments of the genome serve as a fingerprint for the COVID-19 virus. Using this as a basis, scientists have designed a test to unequivocally identify the COVID-19 virus.
By now, either you have personally experienced or seen on TV a trained healthcare worker attired in PPE inserting a plastic swab tipped with artificial cotton wool into the nose or throat. The swab has a long shaft, and it is gently scraped around the back of the nose or upper part of the throat (nasopharynx region). This can be an uncomfortable experience particularly for children. This is the sampling process to conduct a test for the COVID-19 virus. The swab is immediately put into a tube with chemicals, sealed, labelled and sent to a laboratory.
Of the many tests available, two are currently used in Sri Lanka. These are the PCR test and the Rapid Antigen Test. They differ in their sensitivity, specificity, cost and rapidity of results. The PCR test is conducted in a centralised laboratory, while the Rapid Antigen Test can be carried out on the spot.
PCR test
The PCR test is used to diagnose if a patient is infected or not with the COVID-19 virus. It is performed on patients with symptoms or on those who do not show any symptoms but are suspected of having an infection. It is vital that the test is highly sensitive and does not miss a patient infected with the virus (called a false negative result). The test should also be very specific to the COVID-19 virus; it should not diagnose a patient who is not infected with the COVID-19 virus as positive (called a false positive result). The PCR test is able to detect very low virus numbers in the patients. The results usually take around 12 to 48 hours.
Back to the sampling. The stuff on the swab needs to be cleaned. The RNA of the COVID-19 virus should be isolated from the rest of the other stuff that was scraped out from the back of the throat. There would be other bacteria and viruses, cells from our throat and mucus. These would have their own DNA and RNA. A combination of chemicals and detergents are used to clean up the sample and also to break open the COVID-19 virus to release its RNA, which is required for testing. Once this is done the sample is loaded into the PCR machine with another set of chemicals.
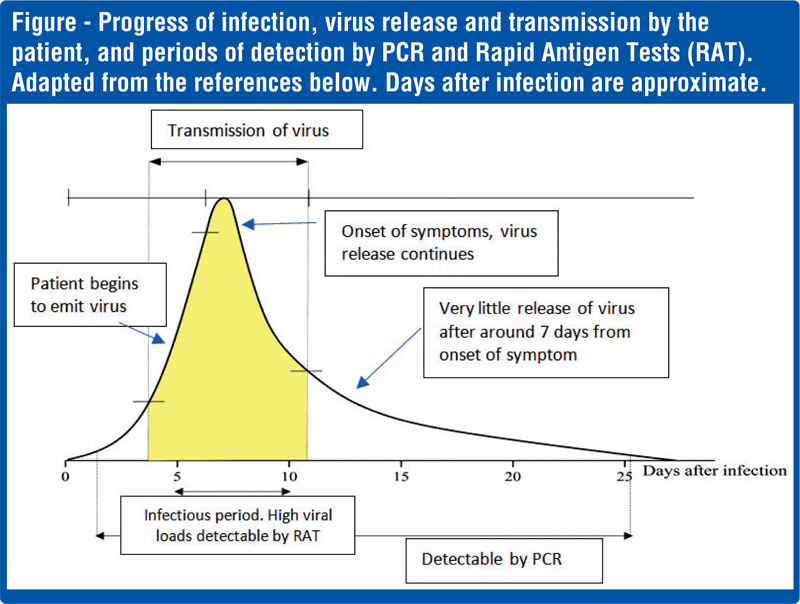
The PCR test is a very accurate and a nearly foolproof test for the presence or absence of the COVID-19 virus. It requires trained laboratory personnel, a modern laboratory, expensive chemicals and equipment, and time usually one or two days depending on the workload. Since PCR testing is very sensitive, it can detect the shedding of the virus from the patient even after the incubation period, and positive results can be given up to 17 days (see the figure).
The incubation period is the time from exposure to the virus to onset of symptoms, which according to the WHO is on average 5-6 days but can be as long as 14 days. However, these PCR positive patients are no longer infectious and hospitalising or quarantining them is a waste of hospital resources and agony for the patients. The WHO recommends that patients be discharged based on clinical recovery and not on a negative PCR.
It is important to note that the PCR test detects the viral RNA fragments, and not the virus capable of causing infections. Thus, a positive PCR does not necessarily mean that a person has infectious virus and is capable of transmitting the virus to others.
Rapid tests for the COVID-19 virus
With a rapid surge in the numbers of infected persons, rapid tests are necessary to prevent the pandemic getting out of control. An on-the-spot testing method is necessary to decide if a bus load of people should be allowed to travel from a region with infected persons to a region which is relatively free of the COVID-19 virus. For this purpose, rapid tests have been developed that give results within 15 to 30 minutes. Similar to sampling for the PCR tests, here too a nasal or throat swab is mixed with chemicals on a paper strip to produce a colour reaction.
There are two different rapid tests for the COVID-19 virus. One is the Rapid Antigen test and the second is the Antibody test. Antigens are proteins found on the surface of the virus; being part of the virus a swab from the nose or throat will detect the virus. Antibodies are produced by our body against the virus and found in the blood, which needs a blood sample for testing. This test would tell us if our body has developed antibodies to combat the virus.
How does the Rapid Tests differ from the PCR?
The PCR test looks for a specific fragment of the COVID-19 viral RNA taken from the patient. Even if this is present in very small amounts the PCR machine multiplies them to a high number of copies. The Rapid Antigen Test looks for specific proteins on the surface of the virus. These proteins are called antigens, used in some vaccines and also recognised by our immune system to launch the defence against the virus.
Unlike the PCR test, the antigens are not multiplied to sufficient levels for the test to detect the virus by the Rapid Tests. They act on the available load of the virus in the sample. The viral load in an infected person is the amount or number of virus particles in the body. Thus, if the virus load in the sample is low, the test can be negative – called a false negative. Obviously, these tiny virus particles cannot be counted; they are labelled as high, medium, or low viral loads. The progress of the viral load with time is shown in the figure.
Sensitivity of the tests
Sensitivity refers to how well a test is able to detect the virus – or specifically the RNA or proteins produced by the COVID-19 virus. The need for sensitivity is, however, different on what our objectives are. If the need is to diagnose a patient at the beginning of an infection cycle (see figure), then the gold standard is the PCR. If the need is to screen the population (many individuals), sensitivity is less of an issue: what is at stake is how infectious are the persons being tested. In other words, do these people have a high viral load with which they can transmit the virus? The RAT is ideally suited for this purpose: it detects high viral loads (hence infectious), many individuals can be screened, it is cheap, and results are available in 15-20 minutes. Thus, the primary need is not to determine if a single person with a small viral load can be accurately identified, but how efficiently infectious people can be detected in a population, who are capable of transmitting the virus to others. Thus, this would help the epidemiologist to isolate and remove infected persons and break the transmission chain. This could be people who are infected and also, importantly, those who are infected but do not show any symptoms, called asymptomatic, and those who are at the beginning of the infection cycle (see figure).
With PCR, there is a time frame from the point of sampling to the release of results during which the infection can spread. Infected persons can also spread the virus before symptoms appear. Those who do not show symptoms – asymptomatic – would also spread the virus. In this context, it is necessary to reduce the period between testing and confirmation of the results, which is not possible with PCR testing.
For the public it is important to note that a negative test result does not necessarily mean one is free of infection. If the test was performed at a point in the infection cycle (see figure) when the viral load is low the RAT would give a negative result.
Implementing the RAT more frequently, is an important tool for the epidemiologist to keep track of the spread of the virus and immediately implement isolation measures. An understanding of the infection cycle of the virus is necessary.
 False negatives
False negatives
What is of concern to the epidemiologist are false negative results – the person has the virus, but the test gives a negative result. This can happen if the Rapid Antigen test is done during the incubation period. During this period there may be insufficient viral proteins (antigen) in the nose or throat. The viral proteins are in sufficient amounts around one-two days before symptoms are seen.
False negatives, with PCR and RAT, can also result from incorrect sampling, if the swabs are not inserted properly and swished around in the nose and throat so that enough viral proteins or virus particles stick onto the swab. This can give a false sense of security or assurance to the person who may go around spreading the virus.
Implications for interventions
The roll out of effective vaccines would not necessarily end the pandemic. This is due to the challenges of successfully vaccinating the entire population and the resurgence of new variants with increased transmissibility, which was not anticipated earlier. In addition, there is asymptomatic transmission, and an overwhelmed health sector that is unable to attend to routine health needs of the people. Lockdowns and closures to reduce social interaction affects individual and government revenue.
Hence, there is an urgent need for an early warning system on the spread of the virus in the population to deploy interventions by the state and prevent the uncontrolled spread of the virus. At present, monitoring of the virus spread is based on daily reports of PCR results, hospital admissions and random Rapid Antigen tests. This, however, does not reflect the prevalence of the virus in the broader community.
The UK implemented a community-wide program to detect the resurgence of the virus at low prevalence in 2020 over six months (see Riley at al. in references). This was a real-time, country-wide population-based surveillance, that can be modified and conducted in Sri Lanka to monitor the COVID-19 virus and provide early warning. This could avoid sudden lockdowns and the inconveniences to the state, economy and the public.
References
McCartney M, Sullivan F, Heneghan C. Information and rational decision-making: explanations to patients and citizens about personal risk of COVID-19. Evidence-Based Med, 2020. [Epub ahead of print.], doi:10.1136/bmjebm-2020-111541.
Crozier, A., Rajan, S., Buchan, I., & McKee, M. (2021). Put to the test: use of rapid testing technologies for covid-19. bmj, 372. https://doi.org/10.1136/bmj.n208
He, X., Lau, E.H.Y., Wu, P. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 26, 672–675 (2020). https://doi.org/10.1038/s41591-020-0869-5
Mina, M. J., Parker, R., & Larremore, D. B. (2020). Rethinking Covid-19 test sensitivity—A strategy for containment. New England Journal of Medicine, 383(22), e120.
Guglielmi, G. (2021). Rapid coronavirus tests: a guide for the perplexed. Nature, 590(7845), 202-5.
Riley S. et al. Resurgence of SARS-CoV-2: detection by community viral surveillance. Science. 2021 May 28; 372(6545):990-5.
(The writer is an Associate Research Professor, Plant and Environmental Sciences, at National Institute of Fundamental Studies, Kandy. He can be reached via: [email protected].)