Wednesday Feb 25, 2026
Wednesday Feb 25, 2026
Tuesday, 23 March 2021 00:00 - - {{hitsCtrl.values.hits}}
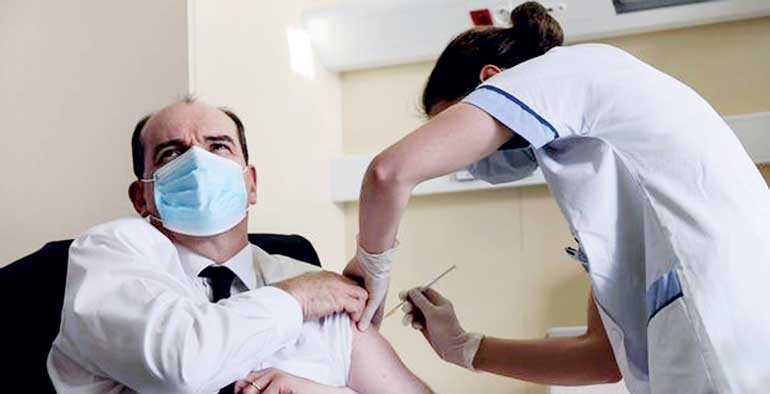
In a possible move to allay the confused messages that are going out, both the British and French Prime Ministers received their first doses of Oxford-AstraZeneca vaccine on Friday 19 March and publicly encouraged others to do so. Pictured is French PM Jean Castex receiving the vaccine
 The Oxford-AstraZeneca vaccine is a chimpanzee adenovirus vectored vaccine, which encodes the spike protein. In the past few weeks, real-world evidence of the benefits of this vaccine has become available.
The Oxford-AstraZeneca vaccine is a chimpanzee adenovirus vectored vaccine, which encodes the spike protein. In the past few weeks, real-world evidence of the benefits of this vaccine has become available.
Initial data from England shows the Oxford-AstraZeneca vaccine to be 60% effective at preventing COVID-19 in persons older than 70 years from days 28 to 34 post-first dose and further increasing to 73% from day 35 onwards. Furthermore, in the EAVE II study in Scotland, the Oxford-AstraZeneca vaccine resulted in substantial reductions in the risk of COVID-19 related hospitalisation.
Starting in the first week of March 2021, sixteen EU countries either totally (Bulgaria, Cyprus, Denmark, France, Germany, Iceland, Italy, Norway, Portugal, Slovenia and Spain) or partially (Austria, Estonia, Latvia, Lithuania, and Luxembourg paused the use of a single batch of a million doses) suspended the roll out of the Oxford-AstraZeneca vaccine. The UK, Belgium, Czech Republic, Poland and Ukraine continued to use this vaccine in their respective countries.
Initially, the World Health Organization (WHO) stated there did not seem to be an increased risk of blood clots and advised that vaccinations should be continued. On 16 March, its Chief Scientist mentioned they did not find an association between these blood clot events and the vaccine. On 19 March, the WHO said its global vaccine safety group had examined data on the Oxford AstraZeneca vaccine administered both in Europe and the 27 million doses administered in India.
They concluded that the current data do not suggest any overall increase in clotting conditions after COVID-19 vaccination, and that the rates of the conditions are fewer than expected. According to MHRA (the UK Medicines regulator), more than 11 million doses of the AstraZeneca vaccine has now been administered across the UK, and the number of blood clots reported after having the vaccine was not greater than the number that would have occurred naturally in the vaccinated population.
The MHRA and UK scientists have advised that people continue to get their Oxford-AstraZeneca COVID-19 vaccine when asked to do so. The company AstraZeneca said 37 blood clots (15 were deep vein thrombosis and 22 were pulmonary embolisms) had been reported out of more than 17 million people vaccinated in the EU and Great Britain. They stated that this was much lower than would be expected to occur naturally in a general population of this size. They also stated that the level was similar across other licensed COVID-19 vaccines.
Following an in-depth analysis, the European Medicines Agency (EMA) concluded that the Oxford-AstraZeneca COVID-19 vaccine is both safe and effective and was not linked to an increased risk of blood clots (thromboembolic events). Data from around 20 million people vaccinated with the Oxford-AstraZeneca vaccine in the European Union (EU) and the United Kingdom (UK) were analysed. In their position statement, they mentioned the following: the benefits of the vaccine in combating the still widespread threat of COVID-19 continue to outweigh the risk of side effects.
They also found the number of blood clot events reported after vaccination to be lower than that expected in the general population. They stated there was no evidence of a problem related to specific batches of the vaccine or to particular manufacturing sites.
Following the release of this guidance, several of the EU countries that had suspended the Oxford-AstraZeneca vaccine roll out, have recommenced their programs. France has put in an additional clause, stating the vaccine should be given to those over the age of 55 years (although the EMA did not allude to such a position).
This may cause some confusion as in February 2021, France did not use this vaccine in those over the age of 65 years (stating the effectiveness in this group was unclear despite the European Medicines Agency, World Health Organisation and the Medicines and Healthcare products Regulatory Agency approving the vaccine across all adult age groups).
In a possible move to allay the confused messages that are going out, both the British and French Prime Ministers received their first doses of Oxford-AstraZeneca vaccine on Friday 19 March and publicly encouraged others to do so. The precautionary principle is very important when evaluating the safety of medicines and vaccines. However Prof Sir David Spiegelhalter from the University of Cambridge, clearly points out the dangers of prematurely making causal links between events. The false rhetoric that follows such incorrect links may damage global vaccine rollout and thus allow the devastating pandemic to progress further.
It is vital for countries to use information appropriately and correctly, as otherwise it risks causing significant harm to effective and coordinated vaccine roll outs. At the present time, there is deep concern of the risk this temporary suspension may have on the third COVID-19 wave affecting certain parts of Europe.
Currently, the risks from COVID-19 continue to be a global cause for concern with nearly 60,000 persons dying from this weekly. The roll out of effective vaccines is the best long-term answer to the current COVID-19 pandemic. A person willing to receive a vaccine would be influenced by its perceived safety. Thus vaccine program suspensions for the incorrect reasons (even if they are temporary), could have long lasting negative effects on public confidence.