Saturday Feb 28, 2026
Saturday Feb 28, 2026
Friday, 27 December 2019 00:00 - - {{hitsCtrl.values.hits}}
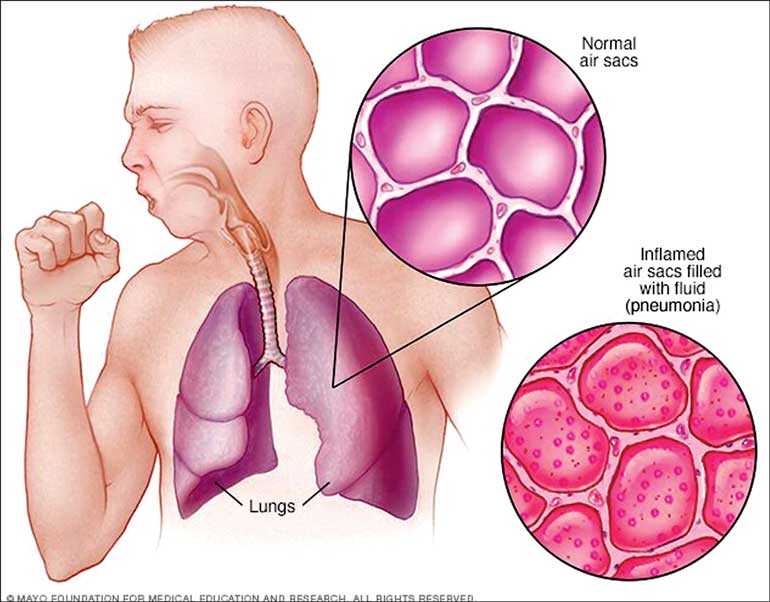
Pneumosil, a vaccine against a leading cause of deadly childhood pneumonia – the pneumococcus bacterium – has achieved prequalification by the World Health Organization (WHO).
Developed though a collaboration spanning over a decade between Serum Institute of India Ltd. (SIIPL) and PATH and with funding from the Bill & Melinda Gates Foundation, the vaccine is expected to provide protection for children on par with other pneumococcal conjugate vaccines at a price that is more affordable for low- and middle-income countries.
Prequalification allows Pneumosi to be procured by United Nations agencies and Gavi, the Vaccine Alliance. This news marks an important milestone toward alleviating one of the biggest barriers to sustainable access to pneumococcal conjugate vaccine that countries face – price.
“Pneumosil can be manufactured at high volume with current capacity at 100 million doses per annum, which will be augmented to over 150 million doses per annum in two to three years. It will be made available at a target Gavi price of around $ 2 per dose, which is roughly 30% less than the current Gavi price for these kinds of vaccines,” says Dr. Rajeev Dhere, executive director of SIIPL.
“The vaccine provides an alternate vaccine for low- and middle-income countries to ensure lifesaving access to pneumococcal disease prevention over the long term,” explains Dr. Mark Alderson, director of PATH’s pneumococcal vaccine project.
Pneumococcal conjugate vaccines have helped significantly reduce pneumococcal deaths and illness where introduced but are difficult for many countries to afford without considerable donor financial support. Although such support has been instrumental in helping to expand country access, implementing and sustaining pneumococcal vaccine programs continues to be difficult for many countries due to the vaccine price. Pneumosil addresses a need for a more affordable option that can make access easier for nations and free up country and donor funds for other health priorities.
“There has been unprecedented demand for the pneumococcal vaccine from Gavi-supported countries,” says Dr. Seth Berkley, CEO of Gavi, the Vaccine Alliance. “Within the last decade, we have supported the vaccination of 185 million children against this leading killer of children – but there is still work to be done. This new vaccine gives us more options in the fight to ensure no child dies from this preventable and treatable disease.”
“Vaccines are one of the best investments we can make to give every child a healthy start at life and build stronger communities and economies,” said Dr. Keith Klugman, director of the pneumonia team at the Bill & Melinda Gates Foundation. “As pneumonia continues to be the leading cause of death for young children worldwide, we welcome this new vaccine that will allow for more children to be protected from this debilitating disease.”
In addition to pneumonia, the pneumococcus bacterium causes other serious, life-threatening diseases like meningitis and sepsis. It is estimated to cause nearly 400,000 deaths in children under five years of age each year worldwide.
Pneumosil covers the types of the bacterium most likely to cause serious illness in low- and middle-income countries. Its manufacturing process has been optimised to make it more efficient – reducing costs while preserving vaccine quality and making the vaccine a more affordable option for countries with the highest burdens of pneumococcal disease.
“Pneumococcal conjugate vaccines are among the most complex vaccines to manufacture. As a vaccine developed and produced in India that will benefit the world, Pneumosil’s WHO prequalification is a landmark achievement,” adds Neeraj Jain, PATH country director in India.
Prior to WHO prequalification, a recent PATH-sponsored Phase 3 clinical study of Pneumosil in infants in The Gambia was conducted at the MRC Unit in The Gambia – part of the London School of Hygiene and Tropical Medicine. The study provided the pivotal results for the data package required for WHO prequalification. In the study, the vaccine met all primary and secondary endpoints with key findings showing the vaccine to have an acceptable safety profile and to generate a protective immune response similar to a WHO-prequalified comparator pneumococcal vaccine without interfering with other routine childhood immunisations. These results were presented at the 2019 European Symposium on Pediatric Infectious Diseases and a study manuscript is in preparation for peer-reviewed publication. Other study collaborators included FHI Clinical and the WHO Reference Laboratory for Pneumococcal Serology at the Great Ormond Street Institute of Child Health, University College London.
Serum Institute of India Ltd. (SIIPL), based in Pune, India, today is the largest manufacturer of diphtheria, tetanus, pertussis (DTP) and measles, mumps, rubella (MMR) vaccines and supplies its products to United Nations agencies and more than 160 countries. SIIPL’s MenAfriVac meningococcal serogroup A conjugate vaccine, which was specifically developed for sub-Saharan Africa, is acknowledged as a remarkable success by the vaccine community. It is a policy and commitment of SIIPL to make new vaccines available at affordable prices to the children of the developing world.
PATH is a global organisation that works to accelerate health equity by bringing together public institutions, businesses, social enterprises, and investors to solve the world’s most pressing health challenges. With expertise in science, health, economics, technology, advocacy, and dozens of other specialties, PATH develops and scales solutions – including vaccines, drugs, devices, diagnostics, and innovative approaches to strengthening health systems worldwide.